Atrial fibrillation, or A-fib, is an irregular heart rhythm that increases the risk of stroke, heart failure and even premature death.
Although many risk factors contribute to fibrosis A, one stands out for its increasing prevalence worldwide: obesity.
I am a cardiology researcher and my team and I have discovered key mechanisms contributing to the detrimental effects of obesity on cardiac function. Targeting these processes may offer new avenues for treatment.
How obesity stresses the heart
Obesity is much more than a weight problem: it fundamentally changes the body’s biochemistry. This in turn changes your metabolism, or the chemical reactions that allow the body to function.
In particular, the increased presence of fatty acids, characteristic of obesity, puts additional pressure on heart cells.
Fatty acids can directly damage heart cells by triggering increased production of reactive oxygen species – specific molecules that can damage tissue and disrupt normal cellular functions.
These effects cause a disruption in the heart’s electrical signals, leading to irregular beats.
allowfullscreen=”allowfullscreen” frameborder=”0″>
One of the main sources of reactive oxygen species is NOX2, an enzyme that becomes more active in obese people. NOX2 influences the activity of several key proteins that regulate heart rhythm. It also contributes to oxidative stress in the atria – the upper chambers of the heart – by changing their size, shape and function. This cardiac remodeling is a key factor in irregular heart rhythms.
A 2-pronged approach
My team and I sought to better understand how NOX2 affects the heart and whether targeting it could reduce the risk of developing an irregular heartbeat in obese people.
We approached this problem from two angles.
First, we used mice fed a high-fat diet to induce obesity similar to humans. One group of these mice was able to produce functional NOX2 while another group could not. We tested drugs that specifically inhibit NOX2 and checked the likelihood of the upper chambers of the heart developing irregular electrical rhythms.
In parallel, we generated human atrial cardiac cells from stem cells and treated them with fatty acids to simulate the effects of obesity. While our mouse model would allow us to capture the effects of obesity on the body as a whole, our cellular model would allow us to study how it affects the heart at the cellular level.
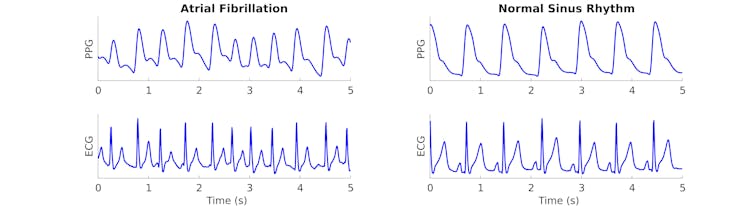
We observed that increased NOX2 activity in obese mice and in treated human cardiac cells led to significant changes in the electrical properties of the heart. Obese mice without NOX2 had less severe atrial fibrillation than obese mice with NOX2.
Similarly, obese mice treated with NOX2 inhibitors also showed improvements in the severity of their irregular heart rhythms, although to a lesser extent. And blocking NOX2 in heart cells exposed to fatty acids reversed the cellular changes caused by fatty acid treatment.
In mice and human heart cells, inhibition of NOX2 reduced oxidative stress and normalized the electrical activity of the heart. This finding suggests that NOX2 plays a key role in the development of obesity-induced irregular heart rhythms.
In particular, we discovered that NOX2 plays an important role in increasing the activity of a gene called PITX2, linked to changes in the electrical function of the heart.
Our data showed that PITX2 activity was enhanced in both obese mice and human heart cells, but reducing NOX2 activity directly lowered PITX2 levels. These results suggest that oxidative stress and genetic factors play a role in the development of irregular heart rhythms.
Treating atrial fibrillation
Current treatments for atrial fibrillation, particularly in obese patients, focus more on managing the symptoms than the physiological changes that cause it.
Although far from reaching the clinical stage, our research suggests that targeting NOX2 could offer a new treatment strategy to prevent or reduce the severity of irregular heart rhythms.
A better understanding of the genetic pathways involved in atrial fibrillation, such as PITX2, could lead to more personalized treatments in the future.
By studying the molecular mechanisms behind irregular heart rhythms, researchers can develop more targeted approaches that address the root causes and improve outcomes for patients suffering from both obesity and heart disease.![]()
Arvind Sridhar, postdoctoral researcher in cardiology, University of Illinois at Chicago
This article is republished from The Conversation under a Creative Commons license. Read the original article.


