Superbugs resistant to existing antibiotics are a growing health problem worldwide. Worldwide, nearly five million people die each year from antimicrobial-resistant infections.
The annual toll of antimicrobial-resistant infections is expected to increase by 70%, with an estimated 40 million deaths by 2050.
To solve this problem, researchers must discover new antibiotics and agents that improve the effectiveness of existing antibiotics.
Hope may come from a surprising source: oysters.
frameborder=”0″allow=”accelerometer; autoplay; write to clipboard; encrypted media; gyroscope; picture in picture; web sharing” referrerpolicy=”strict-origin-when-cross-origin”allowfullscreen>
In new research published today in PLOS ONEwe show that antimicrobial proteins isolated from oyster hemolymph (the equivalent of blood) can kill certain bacteria responsible for various infections. Proteins may also improve the effectiveness of conventional antibiotics against problematic bacterial species.
Robust, resistant bacteria cause common infections
Pneumonia is an acute infection of the lungs, usually caused by Streptococcus pneumoniae. It is the leading cause of death among children under five years of age and a common cause of hospitalization and death among older adults.
Upper respiratory infections, such as tonsillitis, are also common. In fact, this is the most common reason children are prescribed antibiotics.
Persistent skin and throat infections caused by Streptococcus pyogenic can lead to the development of rheumatic fever and rheumatic heart disease.
The high prevalence of these bacterial infections and the overuse of antibiotics have contributed to the evolution of drug-resistant bacteria. This makes these infections difficult to treat.
The formation of biofilms compounds the problem.
Biofilms are populations of millions of bacterial cells embedded in a self-secreted substance that adheres to surfaces. They protect bacteria from the host’s immune system – and from antibiotics. Almost all bacterial infections involve biofilms.
For this reason, new antibiotic treatments capable of inhibiting, disrupting or penetrating biofilms are very valuable.

Oysters as a source of new antimicrobial agents
More than 90% of the antibiotics we currently use come from nature. The same goes for more than 65% of antibiotics under recent development.
In the search for new antimicrobial drugs, researchers usually start by looking at organisms that produce antimicrobial chemicals to defend themselves.
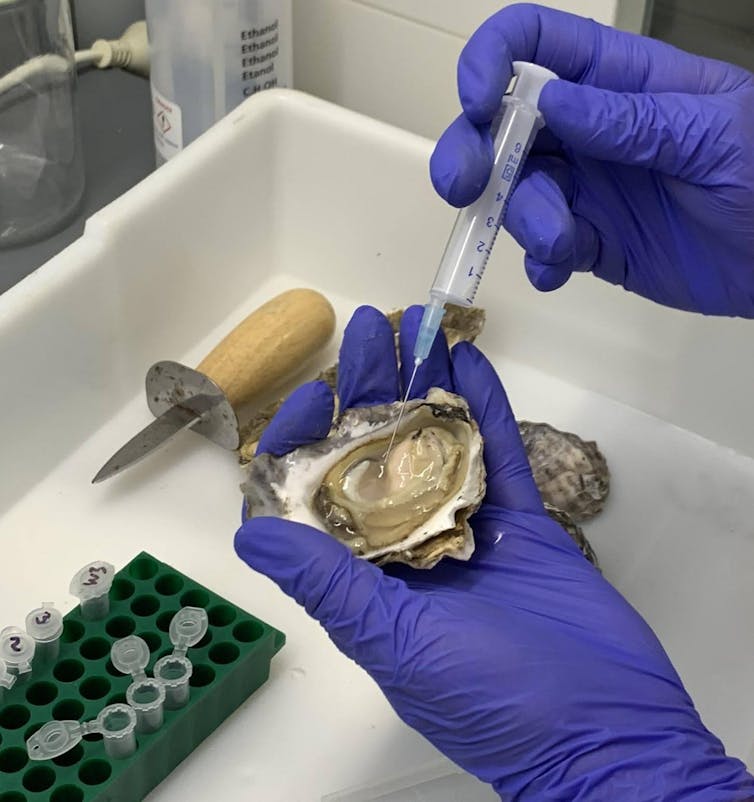
Oysters are exposed to high concentrations of various microorganisms in their natural marine environment. As a result, they have developed strong immune defenses. For example, they rely heavily on antimicrobial proteins and chains of molecules called peptides found in their hemolymph (blood) to protect them from infections.
Research over the past decades has shown that oyster hemolymph contains antiviral and antibacterial proteins and peptides. These are active against a range of human and marine pathogens.
Oysters, along with other molluscs, plants and animals, have long been used as traditional medicines to treat infectious diseases.
In traditional Chinese medicine, various oyster preparations are recommended to treat symptoms of respiratory infections and inflammatory conditions. Oysters have also played an important role in the health of Australia’s indigenous people for millennia. This provides useful clues for drug discovery.
Our latest research confirms that antimicrobial proteins present in the hemolymph of Sydney Pacific oysters (Saccostrea glomerata) are particularly effective in killing Streptococcus spp. bacteria.
Proteins were also effective in inhibiting Streptococcus spp. biofilm formation and could penetrate already formed biofilms.
Strengthening the medicines we have
To improve the effectiveness of currently available drugs, they are increasingly combined with antimicrobial peptides and proteins.
These peptides and proteins can disrupt bacterial cell membranes, helping conventional antibiotics reach their targets more easily. Many of these proteins and peptides can also strengthen the host’s immune system, making treatment even more effective.
We tested the activity of Sydney oyster hemolymph proteins against a range of bacterial pathogens in combination with different commercially available antibiotics. At very low concentrations, the proteins improved the effectiveness of antibiotics by two to 32 times.
The results were particularly promising for Streptococcus spp., Staphylococcus aureus (also known as “Staphylococcus aureus”, a leading cause of drug-resistant skin and blood infections) and Pseudomonas aeruginosa (a major problem for immunocompromised patients with cystic fibrosis). There were also no toxic effects on healthy human cells.
And then?
Overall, oyster hemolymph proteins show promise for future development as an antimicrobial therapy. They can kill pathogens embedded in biofilms, work synergistically with conventional antibiotics and are non-toxic.
However, more work is needed, including animal testing and human clinical trials.
Sustainable sourcing of protein for research and medical use is an important consideration, but this is made easier by the fact that Sydney rock oysters are commercially available.
The results of this work present an opportunity for the pharmaceutical and aquaculture industries to collaborate with researchers on the development of new, more effective antibiotics.![]()
Kate Summer, Postdoctoral Researcher, Southern Cross University and Kirsten Benkendorff, Professor, National Marine Science Centre, Southern Cross University
This article is republished from The Conversation under a Creative Commons license. Read the original article.


