Life is based on the storage and transmission of information. In complex organizations, this information exists in two primary forms: genome and epigenome. Although genetic information remains largely unchanged throughout life, epigenome is dynamic, responding to environmental factors and aging.
Aging results from various biological processes, including genetic mutations, cellular damage and a loss of epigenetic information.
Researchers have collected solid evidence that when cells lose their epigenetic instructions, they undergo changes in the expression of genes which cause a loss of cellular identity. This process leads to many age -related diseases, including neurodegeneration, metabolic disorders and cardiovascular conditions.
Cellular aging and reprogramming
A characteristic of aging is cell senescence, a state in which cells cease to divide. Senescent cells contribute to the dysfunction of tissues by releasing inflammatory molecules and reactive oxygen species, accelerating the aging process. Factors such as DNA damage, shortening of telomeres and oxidative stress trigger this state, making it a key target for potential age -overturning therapies.
The ability to reset the age of a cell without causing harmful mutations or cancer has long intrigued scientists. The first experiences in the 1960s demonstrated that adult cell nuclei contained all the information necessary to create a new organization.
This idea gained ground in 2006 when the researchers identified a group of four transcription factors – Oct4, SOX2, KLF4 and C -MYC (OSKM) – capable of converting adult cells into induced pluripotent stem cells (IPSC). These IPSCs could then transform into any type of cell, which increases the possibility of rejuvenating older tissues.
However, the first experiences with OSKM raised concerns. When expressed at high levels, these factors have triggered uncontrolled cell growth, leading to the formation of tumors. Subsequent studies have revealed a safer approach: using a modified set of three factors (OSK) or by applying OSKM in short gusts.
This method has managed to rejuvenate tissues without causing cancer, to restore function in organs such as optic nerve, kidneys and muscles.
Deputy Inversion of Chemical Age
Although genetic approaches are promising, the delivery of the OSK safely to human tissue remains a challenge. Current methods involve the introduction of genetic material through viral vectors or lipid nanoparticles, which both have drawbacks, including high costs and potential immune reactions. Scientists have sought a chemical alternative – molecules that are capable of imitating the effects of OSK without modifying DNA.
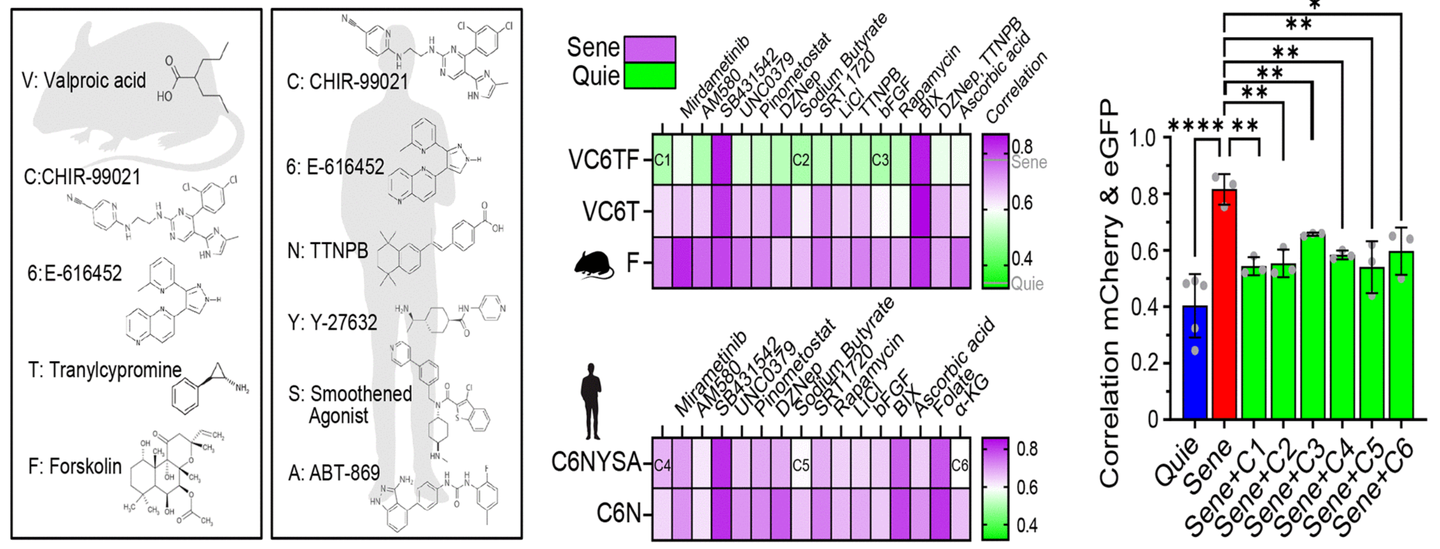
Harvard researchers recently reached an important step in this quest and published their results in the Revue Aging. Using a high -speed screening method, they identified six chemical compounds that have reversed cell aging in human and mice skin cells. These compounds have restored the expression models of young genes and reversed organic age in less than a week.
“This is a breakthrough,” said Dr. David Sinclair, a molecular biologist at the Harvard Medical School and co-author of the study. He thinks that these results mark an important step towards “an affordable rejuvenation of the whole body”.
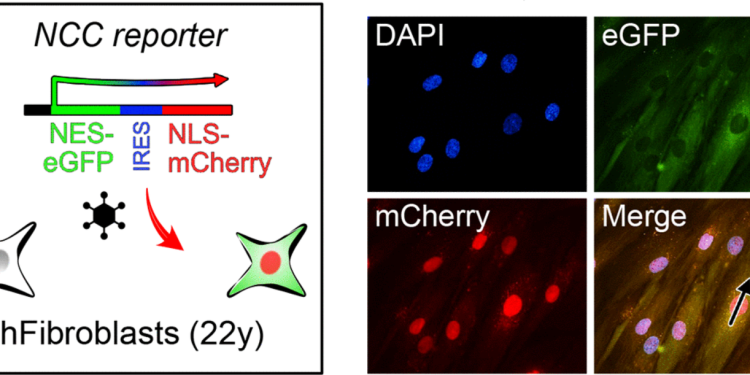
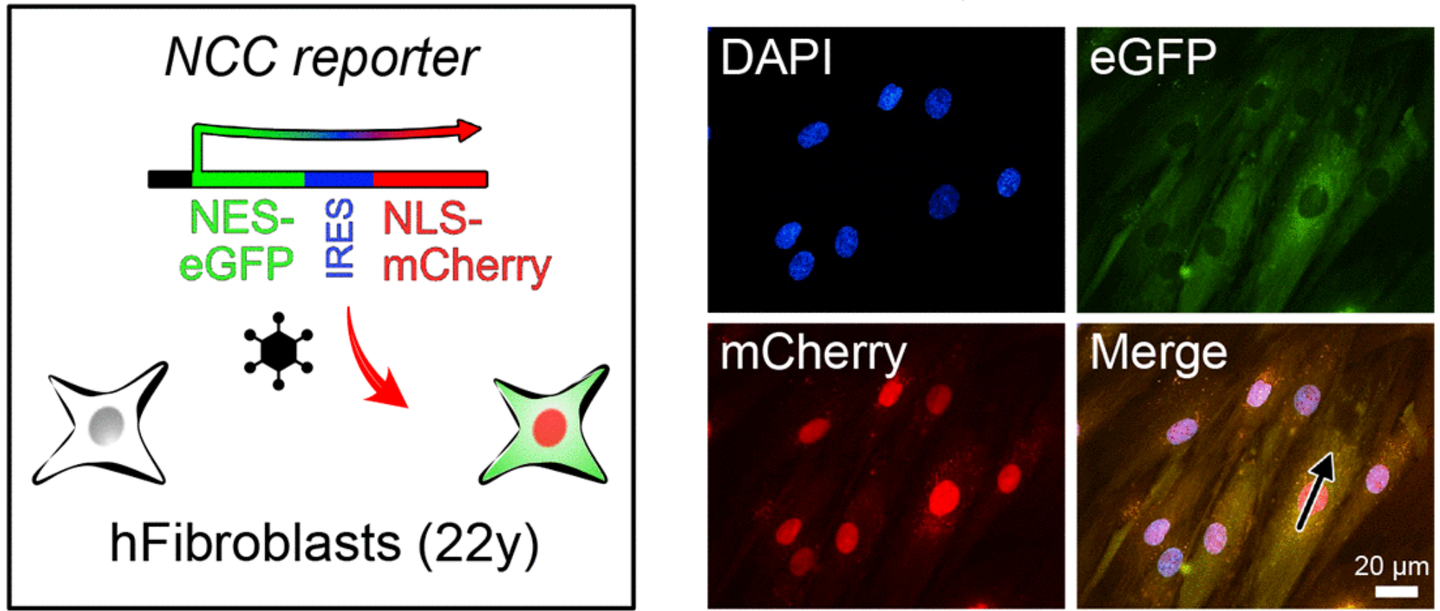
The team used two advanced techniques to measure aging: aging clocks based on transcription and nucleocytoplasmic (NCC) compartmental compartmentalization tests. NCC is a fundamental cellular process that decreases with age, contributing to tissue dysfunction. In restaurants the CCN, the researchers have effectively reversed the key markers of aging.
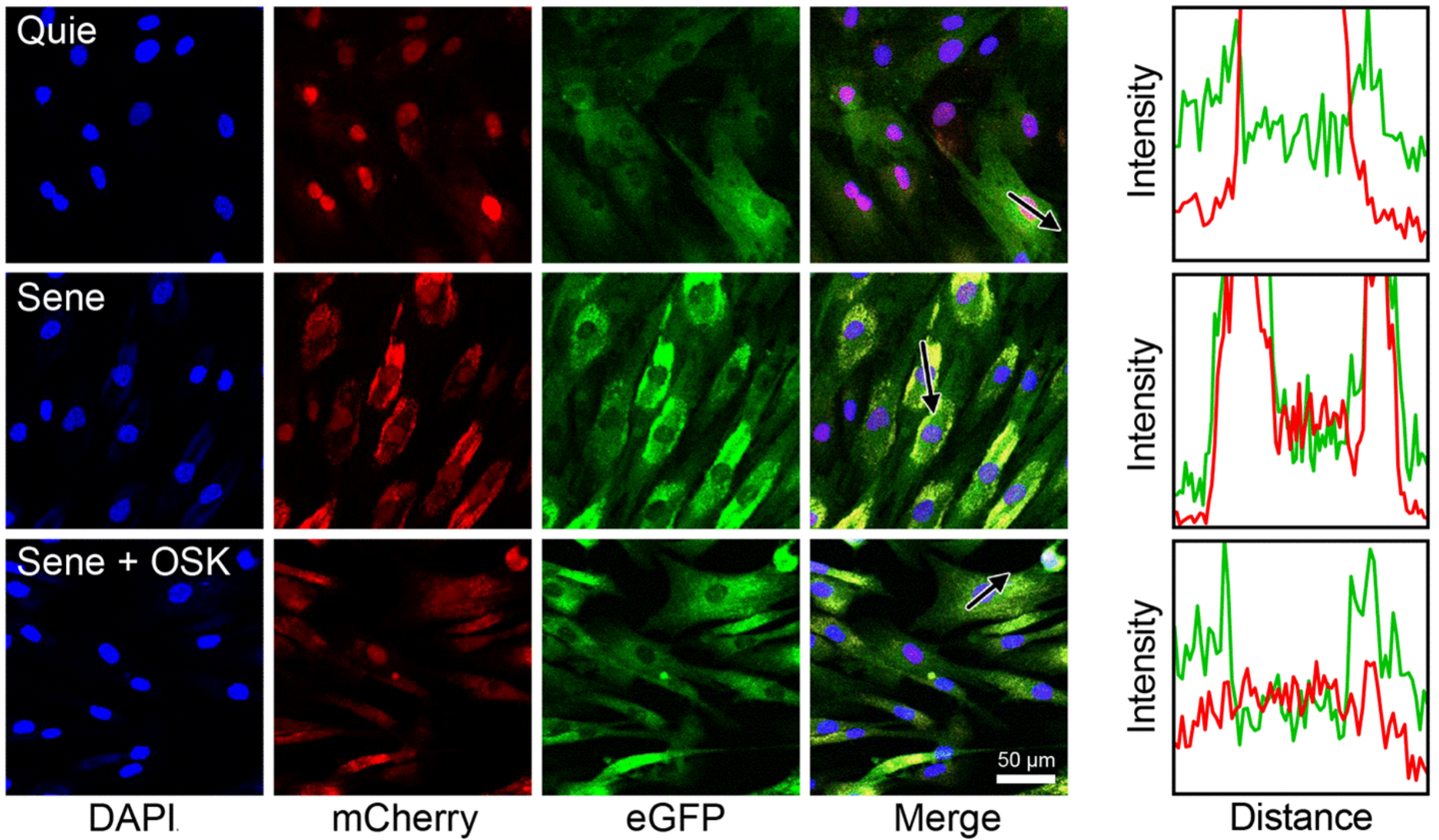
The effects of the compounds were comparable to one year regenerative treatment from a 2019 study which also focused on restoring epigenetic information. “This new discovery offers the opposite potential of aging with a single pill, with applications ranging from improving sight to effectively treat age -related diseases,” projected Sinclair.
Controversy and future challenges
Despite the excitement surrounding the conclusions, some experts urge prudence. Biogerontologist Matt Kaeberlein recognized the potential of the screening method, but argued that the study lacked sufficient validation in animal models. He underlined the need for more in -depth research to demonstrate real advantages, such as improving health or extension of lifespan.
Dr. Charles Brenner, metabolism researcher, raised concerns about three of the study compounds: CHIE99021, which interferes with the metabolism of glycogen; Tranylcypromine, an antidepressant; And valproic acid, treatment of bipolar disorder with potential hepatic toxicity.
“These compounds are generally not sure alone or in combination,” warned Brenner. He also noted that similar chemical approaches had been explored in 2013, suggesting that the allegations of the study were not entirely revolutionary.
Despite these criticisms, research represents a crucial step towards the development of practical therapies for age reversion. Chemical reprogramming offers a safer and more accessible alternative to genetic interventions, potentially opening the way to treatments that restore youth and fight against age -related diseases.
The idea of a “young fountain” has captured humanity for centuries. Although no mythical spring exists to stop aging, science is now close to unlocking the biological mechanisms that govern longevity. With continuous research, the dream of reverse aging can one day become a reality.